

Two lots of Advil Cold & Sinus Day/Night Convenience Pack recalled due to labelling error that may cause confusion between daytime and nighttime tablets.
GlaxoSmithKline Consumer Healthcare ULC (GSK) is recalling two lots of Advil Cold & Sinus Day/Night Convenience Pack (one lot of 18 caplet boxes and one lot of 36 caplet boxes) due to a labelling error on blister packs. The foil backing on the blister pack is rotated upside down and misaligned, so the nighttime caplets are labelled as daytime caplets, and some daytime caplets are labelled as nighttime caplets. Consumers may take a nighttime caplet when they intend to take a daytime caplet, and vice versa.
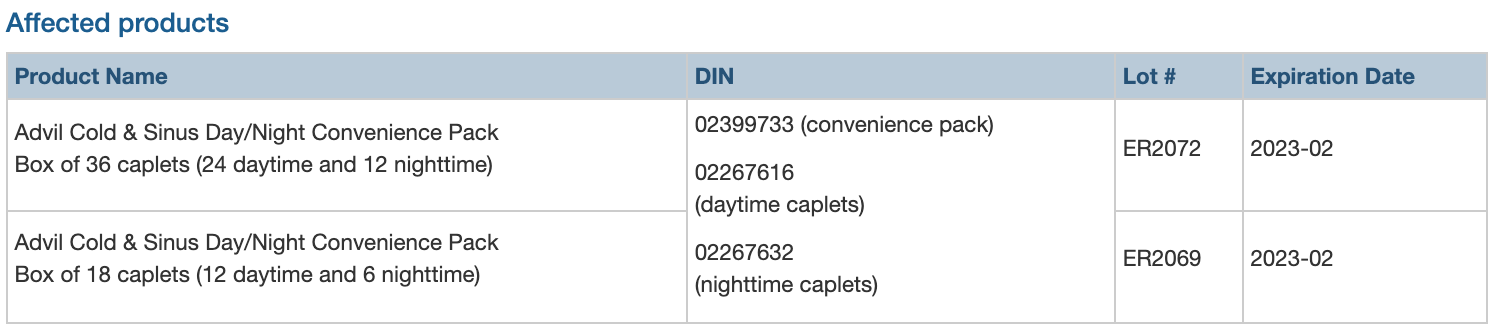
The affected products were distributed in Canada starting July 2021.
Health Canada is monitoring the company’s recall and implementation of any necessary corrective and preventive actions.
What you should do
Stop using the recalled products. Consult a health care professional if you have used any of these products and have health concerns.
- Follow municipal or regional guidelines on how to dispose of chemicals and other hazardous waste; or
- Return the product to your local pharmacy for proper disposal.
Click below to read more information about this recall.
